
Medical Policy
Policy Num: 07.001.171
Policy Name: Laser Surgery of the Prostate for Benign Prostatic Hypertrophy
Policy ID: [07.001.171] [Ac / L / M+ / P+] [0.00.00]
Last Review: October 24, 2024
Next Review: October 20, 2025
07.001.024 Transurethral Radiofrequency Needle Ablation of the Prostate
07.001.047 Cirugía Robótica: Prostatectomía Radical Laparoscopica
07.001.151 - Prostatic Urethral Lift
07.001.169 Temporarily Implanted Nitinol Device (iTind) for Benign Prostatic Hyperplasia
| Population Reference No. | Populations | Interventions | Comparators | Outcomes |
| 1 | Individuals:
| Interventions of interest are:
| Comparators of interest are:
| Relevant outcomes include:
|
| 2 | Individuals:
| Interventions of interest are:
| Comparators of interest are:
| Relevant outcomes include:
|
Description
Benign prostatic hyperplasia (BPH) is a common, noncancerous, and benign enlargement of the prostate gland. BPH is the main cause of significant lower urinary tract symptoms (LUTS) in older individuals with a prostate, including urinary frequency, urinary urgency, feelings of incomplete emptying, having to get up to urinate at night, difficulty starting a urinary stream, or a weak stream that starts and stops. There are multiple means of treatment for the condition, medication being the first line of treatment, and then surgery is considered when all other methods have failed. Symptoms recurrence, and/or complications determine if the patient is a candidate for surgery.
Holmium laser enucleation of the prostate (HoLEP) is an alternative to the gold standard surgery Transurethral Resection of the Prostate (TURP). It is a minimally invasive treatment that does not require an incision. HoLEP is used during a cystoscopy, which guides the laser to access the enlarged tissue of the prostate near the bladder. The laser is used to cut excess tissue out of the prostate gland. It can vaporize, cut through the prostate, and also help to cauterize / coagulate small to medium-sized blood vessels. A significant amount of prostate tissue can be resected with minimal amounts of bleeding. HoLEP has minimal side effects compared to the TURP procedure.
Photo vaporization of the prostate (PVP) is considered an alternative for the Transurethral Resection of the Prostate (TURP). It removes excess prostate tissue by means of vaporization and coagulates the remaining soft tissue using light. PVP has become the reference surgical technique to manage patients who cannot stop anticoagulation/antiplatelet therapy.
For individuals who have benign prostatic hyperplasia and lower urinary tract symptoms who receive Holmium Laser enucleation of the prostate (HoLEP), the evidence includes one 3-month, retrospective study, and one Systematic Review of 10 Randomized Controlled Trials. The outcomes of interest are symptoms, quality of life, and treatment-related morbidity. The main treatment outcomes were measured by IPSS and IPSS Quality of Life Index (IPSS-QoL) at preoperative baseline, then at 1, 3, and up to 12 postoperative months (POM). The holmium laser group had favorable post-operative outcomes in all the studies. A shorter duration of postoperative catheterization and a reduction in the necessity for a 2nd procedure were observed more in the HoLEP, especially in patients with PV > 80 ml. In un-catheterized patients, voiding symptoms and patients’ QoL derived from IPSS and IPSS-QoL clearly significantly and consistently improved after HoLEP. In catheterized patients, the rate of achieving catheter-free status after surgery was significantly higher in HoLEP for patients with PV > 80 ml and PV 30–80 ml. One of the general drawbacks of HoLEP was its prerequisite for morcellation during surgery which subsequently leads to serious operative complications including bladder injury. For both un-catheterized and catheterized patients, improvement in LUTS, achievement of catheter-free status, and the non-necessity of a 2nd procedure were predominant in HoLEP, and these outcomes were more prominent in patients with large BPE of PV > 80 ml. No adverse effects on erectile or ejaculatory function were observed, and improvements were sustained through 5 years of follow-up. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome.
For individuals who have benign prostatic hyperplasia and lower urinary tract symptoms who receive laser photo-vaporization of the prostate (PVP), the evidence includes a systematic review of randomized controlled trials and a retrospective study. The outcomes of interest are symptoms, quality of life, and treatment-related morbidity. The main treatment outcomes were measured by IPSS and IPSS Quality of Life Index (IPSS-QoL) at preoperative baseline, then at 1, 3, and up to 12 postoperative months (POM). Green-light laser photoselective vaporization of the prostate (PVP) had favorable outcomes in all the studies. Analysis revealed PVP is associated with reduced blood loss, transfusion, clot retention, Tur syndrome, capsular perforation, catheterization time, and hospitalization, but also with a higher reintervention rate and longer intervention duration. PVP was shown to have equivalent long-term IPSS, Qmax, QoL, PVR, and IIEF efficacy, and fewer complications than TURP. PVP does not acquire histological tissue examination which removes an opportunity to identify prostate cancer and has a significantly higher incidence of reoperation for persistent/regrowth adenoma present. In the long-term period, showed a higher incidence of reoperation rate due to incomplete vaporization/regrowth of prostatic adenoma. Compared to HoLEP, Qmax, and PVR improved significantly compared to baseline beginning 1 month after surgery in both the PVP and HoLEP groups; this improvement was sustained throughout the 12 months of follow-up. In another study, the increase in the Qmax value 60 months after the PVP was decreased to the baseline level, but there's still a risk of re-operation. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome.
The objective of this evidence review is to determine whether Holmium laser enucleation of the prostate (HoLEP) or Laser photo-vaporization of the prostate (PVP) improves the net health outcome in individuals with benign prostatic hyperplasia.
The use of Holmium laser enucleation of the prostate (HoLEP) or Laser photo-vaporization of the prostate (PVP) in individuals with moderate-to-severe lower urinary tract obstruction due to benign prostatic hyperplasia as an alternative to open prostatectomy or transurethral resection of the prostate may be considered medically necessary when all of the following criteria are met:
• The patient has persistent or progressive lower urinary tract symptoms despite medical therapy (α1-adrenergic antagonists maximally titrated, 5α-reductase inhibitors, or combination medication therapy maximally titrated) over a trial period of no less than 6 months, or is unable to tolerate medical therapy; AND,
• Prostate gland volume is between Moderate (PV 30–80 ml) and Large (PV > 80 ml) BPE,
• Patient presenting refractory urinary retention, acute and/or chronic renal insufficiency, recurrent urinary tract infection, bladder stones, gross hematuria, or recent prostatitis (within the past year);
• Patient presenting high risk for bleeding, or unable to tolerate a surgical procedure due to cardiac issues; AND,
• Patient has had appropriate testing to exclude the diagnosis of prostate cancer.
Coding
Please see the Codes table for details.
BlueCard/National Account Issues
State or federal mandates (eg, Federal Employee Program) may dictate that certain U.S. Food and Drug Administration-approved devices, drugs, or biologics may not be considered investigational, and thus these devices may be assessed only by their medical necessity.
Benefits are determined by the group contract, member benefit booklet, and/or individual subscriber certificate in effect at the time services were rendered. Benefit products or negotiated coverages may have all or some of the services discussed in this medical policy excluded from their coverage.
Benign prostatic hyperplasia, or BPH, is a condition in which the prostate gland is enlarged due to the expansion of the tissue. Usually, the prostate is the large of a walnut or a golf ball. As the prostate enlarges, the gland presses against the urethra, the bladder walls become thick, and the bladder muscle loses the ability to empty completely (urine retention). Other lower urinary tract symptoms (LUTS), like a weak stream or the need to push or strain, need to be monitored. These are the common problems associated with BPH. Surgery is the last treatment considered when all other methods have failed.
Benign prostatic hyperplasia is common in older individuals, it affects about 50 percent of individuals between the ages of 51 and 60 and up to 90 percent of individuals older than 80. The exact cause is not known, and it’s theorized to be related to hormones, including testosterone and especially dihydrotestosterone (a hormone related to testosterone).
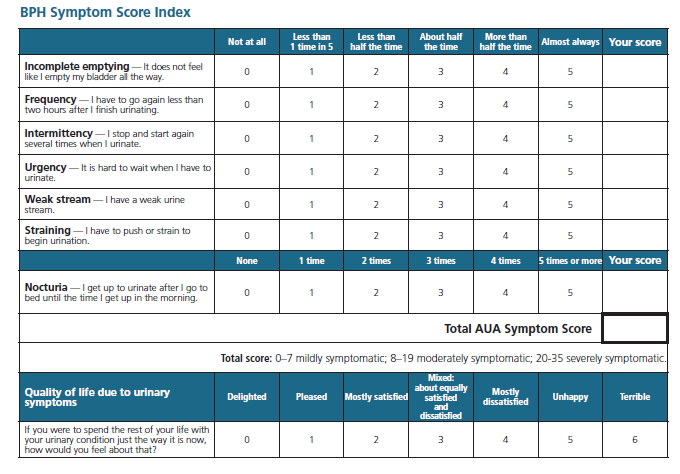
Two scores are widely used to evaluate BPH-related symptoms: the American Urological Association Symptom Index (AUASI) and the International Prostate Symptom Score (IPSS). The AUASI is a self-administered 7-item questionnaire assessing the severity of various urinary symptoms.2, Total AUASI scores range from 0 to 35, with overall severity categorized as mild (≤7), moderate (8-19), or severe (20-35).1, The IPSS incorporates questions from the AUASI and a quality-of-life question or a "Bother score."3.
Treatment is not necessary unless BPH causes bothersome symptoms or complications such as urine tract infections, impaired kidney function, blood in the urine, kidney stones, and urine retention
Alpha-adrenergic blockers (such as terazosin, doxazosin, tamsulosin, alfuzosin, or silodosin) are used to relax certain muscles of the prostate and bladder outlet so that they may improve the flow of urine. Alpha-blockers work instantly. Some reported side effects include dizziness, lightheadedness, fatigue, and difficulty ejaculating. Individuals with moderate to severe BPH and those bothered by their symptoms are good candidates. Drugs such as 5-alpha reductase inhibitors are pills that can increase urine flow and shrink the prostate by blocking DHT (such as finasteride and dutasteride). Alpha reductase inhibitors block the effects of the male hormones responsible for the prostate’s growth, shrinking the prostate and preventing or delaying the need for surgery or other treatments. This can cause some side effects that can include erectile dysfunction and reduced libido (sex drive). Pharmacological treatment may need to be taken for 3 months for the patient to experience relief or may require more than one medication such as an alpha-adrenergic blocker plus either finasteride or dutasteride.
Both an alpha-blocker and a 5-alpha reductase inhibitor can be used together. Two drugs may have more side effects than taking just one. An urologist may add antimuscarinics for patients with overactive bladder symptoms (bladder muscles squeeze uncontrollably), to control the frequent and urgent need to pass urine. This also can lead to incontinence (leaking) when the antimuscarinics relax the bladder muscles.
Individuals with larger prostates can benefit from this treatment.
This list by the American Urological Association enumerates all procedures from least to most invasive that were individually compared with TURP at the time they were developed, which provided a general benchmark for evaluating those procedures. The AUA recommends surgical intervention for patients who have "renal insufficiency secondary to BPH, refractory urinary retention secondary to BPH, recurrent urinary tract infections (UTIs), recurrent bladder stones, or gross hematuria due to BPH, and/or with lower urinary tract symptoms (LUTS) attributed to BPH refractory to and/or unwilling to use other therapies."
These procedures can be performed in an inpatient/outpatient setting. They require minimal to no hospital stay. Recovery is fast and the relief from the symptoms is instantaneous. They do not reduce the risk of additional procedures or pharmacological treatments. The effects may be temporary.
Uses needles to place implants in the prostate. These implants lift and compress the enlarged prostate so that it no longer blocks the urethra. Reference Policy 07.001.151.
It combines radiofrequency energy and water to create steam. The needle and steam cause rapid cell death. The body’s natural healing response then breaks down and removes the dead tissue, causing the prostate to shrink. Reference policy 07.001.011.
A urologist inserts a catheter through the urethra to the prostate. An “antenna” then sends microwaves through the catheter to heat parts of the prostate. This destroys enlarged tissue. A cooling system protects the urinary tract from heat damage.
Catheterization uses a tube called a catheter in the bladder to drain urine. High risk for infections.
Incisions are made where the prostate and the urethra meet, to widen the urinary channel and allow urine to pass more easily.
Vaporization of prostate tissue by a high-powered laser. The procedure is useful for individuals with large prostates, a high risk of bleeding, or cardiac issues.
The Gold Standard of invasive procedures, TURP uses electric current or laser light to cut and remove tissue. A resectoscope is inserted through the penis to provide light, irrigating fluid, and an electrical loop. The loop cuts tissue and seals blood vessels. The removed tissue is flushed into the bladder and out of the body with a catheter. Moderate to severe BPH symptoms are candidates for TURP.
The holmium: YAG laser is a solid-state, pulsed laser that emits light. It combines the qualities of carbon dioxide and neodymium: YAG lasers provide both tissue cutting and coagulation in a single device. Since the holmium wavelength can be transmitted down optical fibers, it is especially suited for endoscopic surgery.
In HoLEP, the surgeon places a thin, tube-like instrument (a resectoscope) through the penis into the urethra. A laser inserted into the resectoscope cuts the excessive tissue with little bleeding. Patients with larger prostates who wish to avoid more-invasive surgery, those with a higher risk of bleeding, and those taking blood-thinning medications, may be good candidates for HoLEP.
ThuLEP is similar to HoLEP but uses a different type of laser.
In TUVP, the surgeon inserts a resectoscope into the urethra with a lens, a light, and a tool that sends out an electrical current to destroy prostate tissue. Heat from the electrical current seals small blood vessels, reducing the risk of bleeding. Reference Policy 07.001.011.
TWJA uses high-pressure water jets to destroy excess prostate tissue and small blood vessels are sealed to reduce the risk of bleeding. Reference Policy 07.001.011.
A simple prostatectomy removes the entire prostate gland with laparoscopic or robotic-assisted surgery. This procedure is only offered to individuals with the largest prostate glands and is usually done using a robotic. This is a long-term cure.
Most patients report improvement after treatment. Depending on the procedure, minimal complications may be present such as infection, bleeding, incontinence, erectile dysfunction, chronic prostatitis, retrograde ejaculation, and/or scar tissue may occur. These effects are temporary. There may be a need for additional treatment or repeat procedures in case of complications or symptoms recurrence.
On March 1999, VersaPulse Select Single Wavelenght (Ho:YAG) and Dual Wavelenght (Ho:YAG/Nd:YAG) surgical lasers and delivery devices and accessories were cleared for marketing by the U.S. Food and Drug Administration (FDA) through the 501 (k) process (K990947). The FDA determined that this device was substantially equivalent to existing devices.
On November 6, 2006, GreenLight HPS TM Surgical Laser System and Accessories were cleared for marketing by the U.S. Food and Drug Administration (FDA) through the 501 (k) process (K062719). The FDA determined that this device was substantially equivalent to existing devices. GreenLight is intended for cutting, coagulating, or vaporizing urologic soft tissues. When used at 532nm it is intended to thermostatically vaporize the prostate tissue of men suffering from benign prostate hyperplasia/hypoplasia (BPH). The device is not intended to treat prostate cancer.
On April 2021, RevoLix HTL (K211534) surgical diode-pumped solid-state laser (DPSS) indicated for use on soft tissue treatments and Lithotripsy in the field of urology, was compared with similar devices: Sphinx jr. Holmium Laser (Primary Predicate Device) (K132975) and RevoLix 200 (Secondary Predicate Device) (K110941) and which are legally marketed Class II medical devices under 21 CFR 878.4810, i.e., laser surgical instruments for use in general and plastic surgery and in dermatology; and for aspects concerning the higher pulse repetition rate of the RevoLix HTL compared to the predicate devices, the Lumenis Pulse 120H (K140388), and Quanta 150W laser (K201455), for pulse mode comparison which is legally marketed Class II medical devices under 21 CFR 878.4810. All of them have been approved for use under the FDA 501 (k) process.
This evidence review was created in June 2023 with searches of the NIH, NICE, and PubMed databases. The most recent literature update was performed through September 30, 2024.
Evidence reviews assess the clinical evidence to determine whether the use of technology improves the net health outcome. Broadly defined, health outcomes are the length of life, quality of life, and ability to function-including benefits and harms. Every clinical condition has specific outcomes that are important to patients and managing the course of that condition. Validated outcome measures are necessary to ascertain whether a condition improves or worsens; and whether the magnitude of that change is clinically significant. The net health outcome is a balance of benefits and harms.
To assess whether the evidence is sufficient to draw conclusions about the net health outcome of technology, 2 domains are examined: relevance, and quality and credibility. To be relevant, studies must represent 1 or more intended clinical use of the technology in the intended population and compare an effective and appropriate alternative at a comparable intensity. For some conditions, the alternative will be supportive care or surveillance. The quality and credibility of the evidence depend on the study design and conduct, minimizing bias and confounding that can generate incorrect findings. The randomized controlled trial (RCT) is preferred to assess efficacy; however, in some circumstances, nonrandomized studies may be adequate. Randomized controlled trials are rarely large enough or long enough to capture less common adverse events and long-term effects. Other types of studies can be used for these purposes and to assess generalizability to broader clinical populations and settings of clinical practice.
Promotion of greater diversity and inclusion in clinical research of historically marginalized groups (e.g., People of Color [African American, Asian, Black, Latino, and Native American]; LGBTQIA (Lesbian, Gay, Bisexual, Transgender, Queer, Intersex, Asexual); Women; and People with Disabilities [Physical and Invisible]) allows policy populations to be more reflective of and findings more applicable to our diverse members. While we also strive to use inclusive language related to these groups in our policies, the use of gender-specific nouns (e.g., women, men, sisters, etc.) will continue when reflective of language used in publications describing study populations.
Laser treatment of benign prostatic hyperplasia has been proven to be an effective tool in the management of Bening Prostatic Hypertrophy. Current laser enucleation techniques available for urology purposes are Holmium-LEP (HoLEP), Thulium-LEP (ThuLEP), Greenlight-LEP (GreenLEP), and Diode-LEP (DiLEP). Various of these techniques have demonstrated similar, or superior post-operative results to the transurethral Resection of the Prostate (TURP), the current gold standard for the management of BPH.
Multiple energy sources and enucleation techniques have now been described. Lasers such as holmium, thulium, potassium titanyl phosphate (KTP), and diode have all their respective followings. There are similarities between the surgical approaches employed using these laser wavelengths, but different tissue interactions make it necessary for some variations to exist. Surgeons familiar with one wavelength and approach cannot necessarily employ the same approach with another laser without putting the patient at risk for injury. Above all else, surgeons must understand basic laser physics in order to use each laser safely and achieve the outcome desired
This policy discusses the photo-selective vaporization (PVP, GreenLight) and holmium-laser enucleation (HoLEP) techniques.
The first wavelength utilized, and longest running, HoLEP has been the most rigorously studied with many randomized trials against TURP and open prostatectomy. Holmium has a 2140 nm wavelength and a high affinity for water. The laser works in a pulsed fashion. The depth of penetration is 0.4 mm. Multiple approaches are utilized, but all rely upon the identification of the surgical capsule and retrograde enucleation along this plane. The original approach utilized incisions at 5 o’clock and 7 o’clock, with enucleation of the middle lobe between the incisions, moving from proximal to the verumontanum to the bladder neck, with the release of the lobe of the bladder neck. The lateral lobes are similarly enucleated along the capsule, moving in a clockwise fashion (the right lateral lobe) or counterclockwise (the left lateral lobe). A 12 o’clock incision is often made to separate the right and left lateral lobes. Energy is not always used to enucleate and most HoLEP practitioners use a combination of blunt dissection with the beak of the scope, and application of energy (Lerner & Rajender, 2015).
One of the newest energies to be utilized for enucleation, KTP has long been used for vaporization and has an extensive following of surgeons. GreenLEP emerged in 2010 and its use has been growing. A combined “vapor-enucleation” approach has also been described. KTP is a 532 nm wavelength laser with a high affinity for hemoglobin. KTP has an optical penetration depth of 0.8mm and a coagulation depth of 1 mm-2 mm. KTP operates in a near-continuous mode. The technique is generally to make a “vaporizing incision” proximal to the verumontanum to identify the surgical capsule. The beak of the scope is then used to mechanically peel the adenoma anteriorly and work clockwise/counterclockwise along the capsule. The laser beam is turned upwards/inwards towards the adenoma, concentrating the energy into the adenoma and away from the capsule. Quick applications of energy can be applied to capsular bleeders (Lerner & Rajender, 2015).
Retrospective Study
A study comparing photo-selective vaporization and holmium-laser enucleation
Baseline characteristics
In comparing the baseline characteristics between the PVP and HoLEP groups, the PVP group had a higher level of serum PSA, a smaller prostate volume, a higher voiding symptom score (VSS), a higher storage symptom score (SSS), a higher total International Prostate Symptom Score (I-PSS), a longer operation time, and greater energy applied during the surgeries than the HoLEP group (Table 1). However, the baseline QoL index was not different between the groups. Regarding the baseline urodynamic data, the HoLEP group showed a smaller postvoid residual urine volume (PVR), a smaller maximum cystometric capacity (MCC), a higher bladder outlet obstruction index (BOOI), a higher bladder contractility index (BCI), and a higher percentage of patients with involuntary detrusor contraction (IDC) than the PVP group (Sun, Yoo, & Park, 2019).
| | Total (n = 1,193) | PVP (n = 439) | HoLEP (n = 754) | P-value |
| Mean ± SD or No. pts (%) | ||||
| Patient demographics | ||||
| Age (yr) | 68.5 ± 7.1 | 68.0 ± 8.0 | 68.7 ± 6.6 | 0.093 |
| BMI (kg/m2) | 24.0 ± 3.0 | 23.9 ± 3.1 | 24.1 ± 3.0 | 0.420 |
| PSA (ng/ml) | 4.2 ± 6.0 | 5.2 ± 8.0 | 3.6 ± 4.1 | <0.001* |
| TPV (ml) | 56.8 ± 28.4 | 51.4 ± 32.0 | 60.0 ± 25.5 | <0.001* |
| Symptom scores | ||||
| Total I-PSS | 19.7 ± 7.6 | 20.6 ± 8.1 | 19.2 ± 7.4 | 0.003* |
| VSS | 11.9 ± 5.2 | 12.3 ± 5.3 | 11.7 ± 5.1 | 0.029* |
| SSS | 7.8 ± 3.5 | 8.3 ± 3.7 | 7.6 ± 3.4 | 0.001* |
| QoL index | 4.2 ± 1.0 | 4.3 ± 1.0 | 4.2 ± 1.0 | 0.207 |
| Uroflowmetric parameters | ||||
| Qmax (ml/s) | 10.4 ± 4.8 | 10.6 ± 5.5 | 10.3 ± 4.6 | 0.548 |
| PVR (ml) | 73.1 ± 102.5 | 83.2 ± 117.3 | 67.2 ± 92.3 | 0.018* |
| BVE (%) | 73.2 ± 24.1 | 72.0 ± 24.5 | 73.9 ± 23.9 | 0.225 |
| Urodynamic parameters | ||||
| FDV (mL) | 187.4 ± 82.5 | 182.1 ± 90.4 | 190.4 ± 77.5 | 0.112 |
| MCC (mL) | 378.8 ± 117.5 | 405.0 ± 89.5 | 365.3 ± 127.5 | <0.001* |
| IDC | 478 (40.1) | 148 (33.7) | 330 (43.8) | 0.028* |
| BOOI | 40.6 ± 27.1 | 35.4 ± 28.2 | 43.7 ± 26.1 | <0.001* |
| BCI | 94.6 ± 30.1 | 88.7 ± 31.4 | 98.1 ± 28.8 | <0.001* |
| Perioperative data | ||||
| Operation time (min) | 69.7 ± 37.9 | 72.1 ± 46.5 | 68.5 ± 32.5 | 0.018* |
| Enucleation ratio | | | 0.78 ± 0.47 | |
| Used energy (joules) | 102.5 ± 63.5 | 129.1 ± 90.8 | 88.7 ± 35.8 | <0.001* |
Post-Operative Outcomes after PVP or HoLEP
In both the PVP and HoLEP groups, the value of the QoL index at each follow-up visit was significantly decreased during the entire follow-up period after surgery compared with that at the baseline (Fig. 1). Additionally, according to the repeated measures analysis of variance (ANOVA) test to adjust for the effect of time on the QoL outcomes, no significant differences were found in the change of the QoL index over time between the PVP and HoLEP groups during the 60-month follow-up period (Fig. S2). The improvement in all outcomes parameters, including total I-PSS, VSS, SSS, the maximum flow rate (Qmax), PVR, and bladder voiding efficiency (BVE), was maintained during the entire follow-up period after PVP or HoLEP, except for Qmax at 60 months after PVP. However, the values of VSS and SSS starting from 36 months after the PVP were increased compared with those at 12 months after surgery, although their decrease was sustained up to 60 months after surgery compared with that at baseline. Meanwhile, the values of VSS starting from 24 months after the HoLEP were increased compared with those at 12 months after surgery, whereas those of SSS at 24, 36, 48, and 60 months after surgery were not different from 12 months postoperatively. The Qmax values in both the PVP and HoLEP groups deteriorated starting from 24 months compared with those at 12 months after surgery. However, the increase in the Qmax value at all follow visits after the HoLEP was maintained up to 60 months compared with that at baseline, whereas the Qmax value at 60 months after the PVP was decreased to the baseline level. The incidence of transient urinary incontinence after HoLEP was higher than that after PVP (Table 2). Repeated BPH surgeries because of the regrowth of prostatic adenoma were performed for 12 patients in the PVP group but for none in the HoLEP group
Serial postoperative outcomes after PVP and HoLEP. The asterisk (*) indicates that, at each follow-up visit, significant differences were found from the value at baseline. A dagger (†) and double dagger (‡) indicate that, at each follow-up visit, a significant difference was found from the baseline value at 1 year and 3 years of follow-up, respectively (Paired t-test, p < 0.05).
Table 2. Comparison of the postoperative complications between PVP and HoLEP
| | PVP | HoLEP | Clavien-Dindo classification |
| Transfusion | 0 (0.0) | 6 (0.8) | II |
| Recatheterization* | 3 (0.7) | 44 (5.8) | II |
| Transient dysuria | 35 (8.0) | 56 (7.4) | I |
| Urge urinary incontinence* | 6 (1.4) | 38 (5.0) | II |
| Stress urinary incontinence* | 1 (0.2) | 175 (23.2) | I |
| Urethral stricture* | 2 (0.5) | 18 (2.4) | IIIa |
| Bladder neck contracture | 1 (0.2) | 7 (0.9) | IIIa |
| Repeated BPH surgery* | 12 (2.7) | 0 (0.0) | IIIa |
PVP = photoselective vaporization of the prostate; HoLEP = holmium laser enucleation of the prostate; BPH = benign prostatic hyperplasia.
Asterisk (*) indicates that there was a statistically significant difference. (Chisquare test or Fisher’s exact test, p < 0.05).
Summary
Sun, Yoo, & Park (2019) conclude that compared with TURP, PVP, and HoLEP showed comparable efficacy and lower postoperative morbidity as mentioned above. PVP provided equivalent efficacy and fewer bleeding complications for 3 years after surgery, compared with TURP. HoLEP was reported to have similar efficacy and lower perioperative complications with a significant level of evidence, than TURP. However, Sun states that there has been a scarcity of studies mainly focusing on QoL improvement after laser prostatectomy. Because the treatment outcomes of BPH surgery greatly affect patients’ QoL, this study may extend the current knowledge regarding them. The results of our study are summarized as follows:
(1) Postoperative improvement of QoL was maintained up to the long-term follow-up period after PVP or HoLEP.
(2) No significant differences were found between the two groups in postoperative changes from the baseline of the QoL index during the 48-month follow-up period after surgery. However, the degree of improvement in QoL at 60 months after HoLEP was greater than that after PVP.
(3) Lower baseline SSS and higher BOOI were independent factors influencing QoL improvement at the short-term follow-up visit after surgery. However, because the follow-up duration was longer, no independent factor influenced QoL improvement at the mid-and long-term follow-up visits after surgery.
Population Reference No. 1
The purpose of Holmium Laser Enucleation of the Prostate (HoLEP) in patients who have lower urinary tract symptoms due to benign prostatic hyperplasia (BPH) is to provide a treatment option that is an alternative to or an improvement on existing therapies such as medical management, transurethral resection of the prostate (TURP), or prostatic urethral lift (PUL).
The following PICO was used to select literature to inform this review.
The purpose of the Holmium Laser Enucleation of the Prostate (HoLEP) in patients who have BPH and LUTS is to provide a treatment option that is an alternative to or an improvement on existing therapies.
The question addressed in this evidence review is: Does laser surgery improve the net health outcome in patients with BPH and LUTS?
The following PICO was used to select literature to inform this review.
The therapy being considered is Holmium laser enucleation of the prostate (HoLEP). Holmium laser enucleation of the prostate (HoLEP) consists of a holmium:yttrium-aluminum-garnet laser source, an end-firing fiber, a continuous-flow resectoscope, continuous saline solution irrigation, and a video system. The procedure begins by first dissecting the median and lateral prostate lobes off of the surgical capsule of the prostate in a retrograde direction from the apex and releasing them into the bladder. At the end of the operation, a Foley catheter is inserted, and the bladder was continuously irrigated. Usually, the patient is discharged after the removal of the Foley catheter on the second day postoperatively.
The following practices are currently being used to treat BPH in this setting:
Conservative treatment, including watchful waiting and lifestyle modifications;
Pharmacotherapy;
Transurethral resection of the prostate (TURP), which is generally considered the reference standard for comparisons of BPH procedures; and
Continued medical management.
The general outcomes of interest are symptoms, functional outcomes, health status measures, quality of life, and treatment-related morbidity.
The International Prostate Symptom Score (IPSS) is used to assess the severity of BPH symptoms. The first seven questions address urinary frequency, nocturia, weak urinary stream, hesitancy, intermittence, incomplete emptying, and urgency each on a scale of 0 to 5. The total score summed across the 7 items measured, ranges from 0 (no symptoms) to 35 (most severe symptoms). A decrease in score indicates improvement.
Quality of life is assessed with various scales including the IPSS-QoL.
Both short-term (up to 12 months) and long-term (12 months and longer) outcomes should be assessed. Treatment-related morbidity can also be assessed in the immediate post-procedure period.
Table 3: Patient-Reported Health Outcome Measures Relevant to Benign Prostatic Hyperplasia
| Measure | Outcome Evaluated | Description |
| American Urological Association Symptom Index; International Prostate Symptom Score | Severity of lower urinary tract symptoms | Patient-administered, 7-item scale. Symptoms rated as mild (0-7), moderate (8-19), or severe (20-35) IPSS asks an additional question, rating QOL as delighted (0) to terrible (6) |
| Benign Prostatic Hyperplasia Impact Index | Effect of urinary symptoms on health domains | Patient-administered, 4-item scale. Symptoms rated as absent (0) to severe (13). |
IPSS: International Prostate Symptom Score; QOL: quality of life
Study Selection Criteria
Methodologically credible studies were selected using the following principles:
To assess efficacy outcomes, comparative controlled prospective trials were sought, with a preference for RCTs;
In the absence of such trials, comparative observational studies were sought, with a preference for prospective studies.
To assess long-term outcomes and adverse events, single-arm studies that capture longer periods of follow-up and/or larger populations were sought.
Hayashi, et.al., (2023), conducted a retrospective study comparing two clinical centers employing second-generation bipolar transurethral vaporization of the prostate (B-TUVP) and holmium laser enucleation of the prostate (HoLEP) for moderate [prostate volume (PV) 30–80 ml] and large (≥ 80 ml) benign prostatic enlargement (BPE). This study enrolled 161 consecutive patients with BPE who underwent B-TUVP in Yokosuka Kyosai Hospital and 286 consecutive patients who underwent HoLEP in Kokusai Shinzen Sougou Hospital. All the B-TUVP and HoLEP procedures in this study were performed between July 2018 and October 2021 at Yokosuka Kyosai Hospital and between September 2016 and March 2021 at Kokusai Shinzen Sougou Hospital. The two centers shared the same surgical indications and management of elevated prostate-specific antigen (PSA) value and anticoagulant/antiplatelet therapy. Surgical indications were International Prostate Symptoms Score (IPSS) > 7, maximum flow rate (Qmax) < 10 ml/s, persistent or recurrent urinary retention, or bladder stones. The patients with PSA ≥ 4 ng/ml were recommended to undergo prostate MRI and if that indicated suspected prostate cancer, they were counseled to undergo needle biopsy before prostate surgery. In the TUVP group with PV > 80 ml, 2 patients were operated on under continuous anticoagulant/antiplatelet therapy, and in the HoLEP group, all patients had stopped anticoagulant/antiplatelet therapy. The B-TUVP and the HoLEP patient characteristics and treatment outcomes were then retrospectively compared. The main treatment outcomes were measured by IPSS and IPSS Quality of Life Index (IPSS-QoL) at the preoperative baseline, then at 1 and 3 postoperative months (POM). IPSS change (%) and IPSS QoL change (%) were calculated by (values at 1 or 3POM / preoperative values) x 100. Shorter operative times and reduced hemoglobin changes were seen in the B-TUVP group than in the HoLEP group in both PV 30–80 ml and > 80 ml. In contrast, a shorter duration of postoperative catheterization and a reduction in the necessity for a 2nd procedure were observed more in the HoLEP group than in the B-TUVP group, especially in patients with PV > 80 ml. In catheterized patients, voiding symptoms and patients’ QoL derived from IPSS and IPSS-QoL clearly and significantly improved after B-TUVP and HoLEP in both PV 30–80 ml and PV > 80 ml, but these improvement rates were consistently greater in HoLEP than in B-TUVP. In catheterized patients, the rate of achieving catheter-free status after surgery was significantly higher in HoLEP than in B-TUVP for patients with PV > 80 ml and PV 30–80 ml. The incidence of postoperative fever was significantly higher in B-TUVP than in HoLEP in patients with PV 30–80 ml but not in PV > 80 ml. The most common surgical complication of both B-TUVP and HoLEP was postoperative fever which was more frequent in B-TUVP than in HoLEP, especially in patients with PV 30–80 ml. Vaporization via B-TUVP dilates blood vessels possibly leading to higher risks of infection than enucleation with HoLEP. One of the general drawbacks of HoLEP was its prerequisite for morcellation during surgery which subsequently leads to serious operative complications including bladder injury. For both catheterized and catheterized patients, improvement in LUTS, achievement of catheter-free status, and the non-necessity of a 2nd procedure were predominant in HoLEP, and these outcomes were more prominent in patients with large BPE of PV > 80 ml. However, B-TUVP resulted in less blood loss, shorter operative duration, and less urinary incontinence in both moderate and large BPE.
Yılmaz, et al., (2023) evaluated in a retrospective study how safe and effective the Holmium Laser Enucleation of the Prostate (HoLEP) surgery when performed in patients with benign prostatic obstruction (BPO) requiring anticoagulant/antiplatelet (AC/AP). The retrospective data of 250 patients who underwent HoLEP between January 2020-May 2022 were included in the study that had an AC/AP treatment status. Two groups were created of those requiring AC/AP (group 1, n=129) and those not using (group 2, n=121). Basic characteristics, preoperative and postoperative IPSS scores, Qmax, and continence status’ at 1st and 6th-month follow-ups were recorded. Intra- and postoperative complications were recorded according to Clavien-Dindo classification. It was concluded that no significant difference was observed between the groups in terms of preoperative characteristics including prostate-specific antigen, hemoglobin (Hb), prostate volume, IPSS, Quality of Life score, Qmax, Qave, and postvoiding residual volume (p>0.05). There was no significant difference between the two groups in terms of postoperative functional parameters and urinary continence (p>0.05) and in Hb drop (0.13±0.1 g/ dL vs. 0.08±0.15 g/dL, respectively; p=0.21). The blood transfusion rate was 2.3% in group 1 and 0.8% in group 2, and there was no significant difference between the groups (p=0.62). Additionally, there was no significant difference between the groups regarding complications. Conclusion: HoLEP is a safe and effective, minimally invasive surgical method that improves functional parameters in BPO patients requiring AC/AP.
A systematic review and meta-analysis of ten randomized controlled trials that included 1,157 participants on the efficacy and safety of holmium laser enucleation of the prostate, compared to the bipolar technologies for the management of BPH, the holmium laser group had favorable perioperative outcomes in this study. The holmium laser group identified shorter catheterization duration and shorter hospital stay duration than the bipolar technologies group. Efficiency outcomes, such as International Prostate Symptom Score, peak urinary flow rate, quality of life, post-void residual urine volume, and international index of erectile function reported no obvious differences between the holmium laser and bipolar technologies groups at the 6 to 12 months follow-up. Bipolar technologies and holmium laser groups shared equivalent effectiveness and safety in treatments for benign prostate hyperplasia. Holmium lasers identified lower catheter times, shorter hospital stays, and lesser risk of hemorrhage than bipolar technologies (Che, Zhou, Chai, Cui, & Zhang, 2022)
Table 3. The Details and Baseline Characteristics of Individual Study.
| Study | Study design | Treatment | Sample size | Postoperative follow-up period | Age (years) | Prostate volume (mL) | IPSS | Qmax (mL/s) | PSA (ng/mL) | PVR (mL) |
| RCT | HoLEP | 73 | 24 months | 65.95 ± 6.76 | 105.8 ± 46.12 (76–295) | 26.53 ± 4.29 (18–34) | 4.54 ± 3.65 (0–11) | 7.6 ± 4.19 (2.2–19) | 155.4 ± 120.3 (24–470) | |
| Bipolar | 72 | 65.59 ± 6.72 | 102.95 ± 19.69 (76–173) | 25.4 ± 2.91 (20–31) | 4.7 ± 3.43 (0–12) | 7.56 ± 3.56 (2.8–17) | 161.36 ± 107.68 (20–410) | |||
| RCT | HoLEP | 60 | 1, 4, 12, 24, 36 months | 66.2 ± 7 | 107 ± 21 | NA | NA | 8.2 ± 4.1 | NA | |
| Bipolar | 62 | 66.1 ± 7 | 106 ± 23 | NA | NA | 8.7 ± 3.8 | NA | |||
| Gu et al. (2018) | RCT | HoLEP | 122 | 6 years | NA | NA | 5.05 ± 0.17 | NA | NA | NA |
| Bipolar | 119 | NA | NA | 5.13 ± 0.17 | NA | NA | NA | |||
| RCT | HoLEP | 30 | 6 months | 60.07 ± 4.51 | 76.5 ± 17.22 (45–110) | 22.56 ± 2.45 (18–27) | 7.39 ± 0.85 | 4.16 ± 1.28 | NA | |
| Bipolar | 30 | 61.20 ± 4.21 | 80.60 ± 17.79(55–120) | 22.17 ± 2.34(19–28) | 6.98 ± 1.02 | 4.39 ± 1.25 | NA | |||
| RCT | HoLEP | 60 | 1 week, 1 month | 60.85 ± 4.03 | 68.15 ± 11.17 | 23.22 ± 1.96 | 6.96 ± 0.89 | 4.48 ± 1.47 | NA | |
| Bipolar | 60 | 60.35 ± 3.93 | 67.2 ± 9.72 | 23.40 ± 2.31 | 6.63 ± 0.95 | 4.60 ± 1.51 | NA | |||
| Y. B. Chen et al. (2013) | RCT | HoLEP | 140 | 1, 6, 12, 24 months | 73.48 ± 8.8 | 128.16 ± 62.11 | 23.27 ± 3.91 | 7.21 ± 2.44 | 2.23 ± 1.27 | 128.16 ± 62.11 |
| Bipolar | 140 | 72.11 ± 7.8 | 131.33 ± 61.85 | 23.63 ± 3.22 | 7.20 ± 2.18 | 2.35 ± 1.52 | 131.33 ± 61.85 | |||
| RCT | HoLEP | 33 | 12 months | 66.81 ± 7.77 | 125 | 25.24 ± 4.87 | NA | NA | 135.37 ± 46.83 (70–204) | |
| Bipolar | 31 | 67. 48 ± 6.46 | 102 | 25.35 ± 4.17 | NA | NA | 159.41 ± 63.16 (80–280) | |||
| RCT | HoLEP | 44 | 6 months | NA | 133 | NA | NA | NA | NA | |
| Bipolar | 44 | NA | 123 | NA | NA | NA | NA | |||
| RCT | HoLEP | 20 | 1, 3, 6, 12 months | 68.9 ± 2.0 | 57.0 ± 5.1 | 25.7 ± 1.3 | 7.4 ± 0.5 | NA | 125.0 ± 19.3 (13–321) | |
| Bipolar | 20 | 67.0 ± 1.7 | 51.0 ± 3.9 | 24.4 ± 1.2 | 7.5 ± 0.8 | NA | 114.0 ± 23.2 (2–366) | |||
| RCT | HoLEP | 52 | NA | NA | 58 ± 8.2 | NA | NA | NA | NA | |
| Bipolar | 46 | NA | 56.5 ± 6.8 | NA | NA | NA | NA |
Note. M ± SD (range). IPSS = International Prostate Symptom Score; Qmax = maximum urine flow rate; PSA = prostate-specific antigen; PVR = postvoid residual; RCT = randomized controlled trial; HoLEP = holmium laser enucleation of prostate; NA = no available; e: enucleation.
Table 4 presents the postoperative complication events. There was no marked difference in transient incontinence, retention of urine, capsule perforation, bladder neck contracture, blood transfusion, and urinary tract infection (UTI) between the bipolar technology and HoLEP groups.
Table 4. Postoperative Conditions.
| Conditions | HoLEP | Bipolar techs | p |
| Transient incontinence | 18/166 | 18/165 | .98 |
| Retention of urine | 0/166 | 1/165 | .5 |
| Capsule perforation | 0/166 | 7/165 | .06 |
| Blood transfusion | 0/166 | 8/165 | .05 |
| UTI | 8/106 | 9/103 | .75 |
| Bladder neck contracture | 1/93 | 1/93 | 1 |
Note. HoLEP = holmium laser enucleation of the prostate; UTI = urinary tract infection.
For individuals who have benign prostatic hyperplasia and lower urinary tract symptoms who receive Holmium Laser enucleation of the prostate (HoLEP), the evidence includes one 3-month, retrospective study, and one Systematic Review of 10 Randomized Controlled Trials. The outcomes of interest are symptoms, quality of life, and treatment-related morbidity. The main treatment outcomes were measured by IPSS and IPSS Quality of Life Index (IPSS-QoL) at preoperative baseline, then at 1, 3, and up to 12 postoperative months (POM). The holmium laser group had favorable post-operative outcomes in all the studies. A shorter duration of postoperative catheterization and a reduction in the necessity for a 2nd procedure were observed more in the HoLEP, especially in patients with PV > 80 ml. In un-catheterized patients, voiding symptoms and patients’ QoL derived from IPSS and IPSS-QoL clearly and significantly and consistently improved after HoLEP. In catheterized patients, the rate of achieving catheter-free status after surgery was significantly higher in HoLEP for patients with PV > 80 ml and PV 30–80 ml. One of the general drawbacks of HoLEP was its prerequisite for morcellation during surgery which subsequently leads to serious operative complications including bladder injury. For both un-catheterized and catheterized patients, improvement in LUTS, achievement of catheter-free status, and the non-necessity of a 2nd procedure were predominant in HoLEP, and these outcomes were more prominent in patients with large BPE of PV > 80 ml. No adverse effects on erectile or ejaculatory function were observed, and improvements were sustained through 5 years of follow-up. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome.
| Population Reference No. 1 Policy Statement | [X] Medically Necessary | [ ] Investigational |
Population Reference No. 2
The purpose of Photo-selective Vaporization (PVP) in patients who have lower urinary tract symptoms due to benign prostatic hyperplasia (BPH) is to provide a treatment option that is an alternative to or an improvement on existing therapies such as medical management, transurethral resection of the prostate (TURP), or prostatic urethral lift (PUL).
The following PICO was used to select literature to inform this review.
The purpose of the Photo-selective Vaporization (PVP) of the prostate in patients who have BPH and LUTS is to provide a treatment option that is an alternative to or an improvement on existing therapies.
The question addressed in this evidence review is: Does laser surgery improve the net health outcome in patients with BPH and LUTS?
The following PICO was used to select literature to inform this review.
The therapy being considered is Photo vaporization of the Prostate (PVP). PVP consists of selective photo-thermolysis (i.e., selective thermal confinement of light-induced damage). Selected wavelengths of laser light are targeted to different constituents of the tissue to ablate the prostate tissue. The KTP (potassium-titanyl-phosphate) laser (e.g., GreenLight laser) uses a wavelength of 532 nm, which is near the peak absorption of blood.
The following practices are currently being used to treat BPH in this setting:
Conservative treatment, including watchful waiting and lifestyle modifications;
Pharmacotherapy;
Transurethral resection of the prostate (TURP), which is generally considered the reference standard for comparisons of BPH procedures; and
Continued medical management.
The general outcomes of interest are symptoms, functional outcomes, health status measures, quality of life, and treatment-related morbidity.
The International Prostate Symptom Score (IPSS) is used to assess the severity of BPH symptoms. The first seven questions address urinary frequency, nocturia, weak urinary stream, hesitancy, intermittence, incomplete emptying, and urgency each on a scale of 0 to 5. The total score summed across the 7 items measured, ranges from 0 (no symptoms) to 35 (most severe symptoms). A decrease in score indicates improvement.
Quality of life is assessed with various scales including the IPSS-QoL.
Both short-term (up to 12 months) and long-term (12 months and longer) outcomes should be assessed. Treatment-related morbidity can also be assessed in the immediate post-procedure period.
Methodologically credible studies were selected using the following principles:
To assess efficacy outcomes, comparative controlled prospective trials were sought, with a preference for RCTs;
In the absence of such trials, comparative observational studies were sought, with a preference for prospective studies.
To assess long-term outcomes and adverse events, single-arm studies that capture longer periods of follow-up and/or larger populations were sought.
Lai, et al., (2018) compared Green-Light Laser photoselective vaporization of the prostate (PVP) against the gold standard transurethral resection of the prostate (TURP) for the management of lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). Randomized controlled trials and prospective studies were selected for comparing the safety and efficacy of PVP versus TURP for LUTS manifesting through BPH. Perioperative parameters, complications rates, and functional outcomes including treatment-related adverse events such as International Prostate Symptom Score (IPSS), maximum flow rate (Qmax), postvoid residual (PVR), quality of life (QoL), and International Index of Erectile Function (IIEF). Twenty-two publications consisting of 2665 patients were analyzed. Pooled analysis revealed PVP is associated with reduced blood loss, transfusion, clot retention, Tur syndrome, capsular perforation, catheterization time, and hospitalization, but also with a higher reintervention rate and longer intervention duration (all p<0.05). No significant difference in IPSS, Qmax, QoL, PVR, or IIEF at 3, 24, 36or 60 months was identified. There was a significant difference in QoL at 6 months (MD=−0.08; 95% CI −0.13to −0.02; p=0.007), and IPSS (MD = −0.10; 95% CI −0.15to −0.05; p<0.0001) and Qmax (MD=0.62; 95% CI 0.06to 1.19; p=0.03) at 12 months, although these differences were not clinically relevant. PVP is an effective alternative, holding additional safety benefits. PVP has equivalent long-term IPSS, Qmax, QoL, PVR, IIEF efficacy, and fewer complications. The main drawbacks are dysuria and reintervention, although both can be managed with noninvasive techniques. The additional shortcoming is that PVP does not acquire histological tissue examination which removes an opportunity to identify prostate cancer.
Castellani, et.al., (2021) in a systematic review and meta-analysis for evaluation of the efficacy of the GreenLight laser™ photo vaporization of the prostate (GLL-PVP) determined that it has become a valid alternative to traditional transurethral resection of the prostate (TURP) in men requiring surgery for benign prostatic hyperplasia. The purpose of the study was to review systematically the safety and efficacy of studies comparing GLL PVP and TURP in the medium term. Twelve studies were identified for meta-analysis. They showed a longer postoperative catheterization time (risk ratio (RR): 1.12, 95% CI:1.09–1.14, p<0.00001) and length of stay (RR: 1.16, 95% CI:1.12–1.19, p<0.00001) in the TURP group; higher risk of transfusion in the TURP group (RR: 6.51, 95% CI: 2,90–14,64 p<0.00001); no difference in the risk of urinary tract infections (RR: 0.83, 95% CI: 0.58–1.18, p=0.30) and transient re-catheterization (RR: 1.11, 95% CI: 0.76–1.60, p=0.60). Regarding reoperation rate, no difference was found in terms of postoperative urethral stricture (RR: 1.13, 95% CI: 0.73–1.75, p=0.59) and bladder neck contracture (RR: 0.66, 95% CI: 0.31–1.40, p=0.28). A significantly higher incidence of reoperation for persistent/regrowth adenoma was present in the GLLL- VP (RR: 0.64, 95% CI: 0.41–0.99, p=0.05). Data at 2-year follow-up showed significantly better post-voiding residual (PVR) (MD: -1.42, 95% CI: -2.01, -0.82, p<0.00001) and International Prostate Symptom Score (IPSS) (MD: -0.35, 95% CI: -0.50, -0.20, p<0.00001) after TURP. No difference was found in the mean PVR at 2 years after TURP, in the mean maximum flow rate (Qmax) (MD: 0.30, 95% CI: -0.02–0.61, p=0.06) and quality of life QoL score (MD: 0.05, 95% CI: -0.02–0.42, p=0.13). At 5-year follow-up, data showed better IPSS (MD: -1.70, 95% CI: -2.45,-0.95, p<0.00001), QoL scores (MD: -0.35, 95% CI: -0.69, -0.02, p=0.04) and Qmax (MD: 3.29, 95% CI: 0.19–6.38, p=0.04) after TURP. Data of PVR showed no significant difference (MD: -11.54, 95% CI: -29.55–6.46, p=0.21). The analysis concluded that GLL-PVP is a safer and more efficacious procedure than standard TURP in the early and medium term. However, in the long-term period, GLL-PVP showed a higher incidence of reoperation rate due to incomplete vaporization/regrowth of prostatic adenoma.
Elmansy, et.al. (2023) determined Holmium Laser Xpeeda Vaporization and GreenLight XPS Vaporization are safe and effective treatments of BPH. In this randomized controlled trial, same-day discharge with early TOV is a feasible option. Ninety-two men with benign prostatic hyperplasia (BPH) and prostate size ≤80 g scheduled for laser prostatectomy were included in this prospective randomized trial. Outcome measures were collected and compared, including International Prostate Symptom Score (IPSS), quality of life (QoL), flow rate, postvoid residual urine volume (PVR), International Index of Erectile Dysfunction (IIEF)-15, prostate-specific antigen (PSA), transrectal ultrasound prostate volume, and catheterization time. Perioperative complications were also recorded. Patients were offered a trial of void (TOV) 3 hours after their procedures. All patients were followed-up at 1, 3, 6, and 12 months. There were no significant differences in preoperative baseline data between the two surgical groups. Operative parameters and postoperative outcomes were comparable. Effective same-day TOV was noted in 73.1% and 72.7% of the Xpeeda and GreenLight XPS patients, respectively (p = 0.98). All patients were discharged home within 24 hours of their surgeries. The laser energy and postoperative complications were significantly lower in the Xpeeda group (p = 0.002 and p = 0.026, respectively). At 3 months, the PSA levels significantly dropped in both groups (p = 0.002 and p < 0.001). There were no significant differences in functional and sexual outcomes between the two groups at 12 months.
Retrospective Study
A 2016 retrospective study by Kim et. al. concluded that there's no significant difference between the PVP and HoLEP group, with the exception of IPSS voiding subscore at 1 month postoperatively (5.9 vs. 3.8, P< 0.001). PCP was performed in 176 patients and 162 were treated using HoLEP at the same facility between May 2009 and December 2014. Peri-operative and post-operative parameters—such as International Prostate Symptom Score (IPSS), quality of life (QoL), maximum urinary flow rate (Qmax), post-void residual urine volume (PVR), and complications—were compared between the groups. Patients with prostate volumes < 40 mL based on preoperative trans-rectal ultrasonography were included in this study. Preoperative demographic data were similar in both groups, with the exception of PVR. The study concluded that both were efficient and safe as treatments for patients with small prostate volumes.
A total of 338 patients (PVP group: 176, HoLEP: 162) with small prostate volumes accorded to the inclusion criteria were included in the study. The demographic and disease characteristics of the patients are presented in Table 1. The mean age of the patients in the PVP and HoLEP groups was 70.7 and 69.5 years, respectively (P = 0.093). The mean prostate volume was similar in the two groups (30.2 vs. 29.2 mL, P = 0.091). There was no significant difference between the two groups with respect to preoperative PSA (2.0 vs. 1.9 mg/mL, P = 0.300), total IPSS score (20.4 vs. 21.5, P = 0.241), IPSS QoL score (4.1 vs. 4.2, P = 0.640), Qmax (8.7 vs. 9.3 mL/s, P = 0.174). However, PVR was larger in the PVP group (133 vs. 86.8 mL, P < 0.001).
Table 1. Demographic characteristics
Table 2 summarizes the perioperative parameters and complications after PVP and HoLEP. There was no significant difference in operative time, or indwelling catheter duration between the groups. However, compared with the HoLEP group, patients in the PVP group had higher total energy usage (91.1 vs. 83.9 KJ, P = 0.041). The incidence of complications was not significantly different between the PVP and HoLEP groups during the follow-up (6.8% vs. 3.7%, P = 0.635). Urethral stricture occurred in 2.3% of patients after PVP and in 2.5% after HoLEP. BNC was observed in six cases (3.4%) in the PVP group and in two cases (1.2%) in the HoLEP group.
Table 2. Perioperative data and adverse events in patients with small prostate volume undergoing PVP or HoLEP
Subjective follow-up data are shown in Table 3. At the 12-month follow-up, compared with preoperative data, significant ameliorations of the total IPSS score, voiding IPSS subscore, storage IPSS subscore, and QoL were observed after the operation (P <0.05). However, the differences between both groups were not significant, with the exception of the voiding IPSS subscore at 1 month postoperatively (5.9 vs. 3.8, P < 0.001). Objective follow-up data, including Qmax and PVR, are shown in Fig 1. Qmax and PVR improved significantly compared to baseline beginning 1 month after surgery in both the PVP and HoLEP groups; this improvement was sustained throughout the 12 months of follow-up.
Table 3. Follow-up data of up to 12 months after PVP or HoLEP
Qmax, urinary peak flow rate; PVR, post-voiding residual urine; PVP, photoselective vaporization of the prostate; HoLEP, holmium laser enucleation of the prostate. * P < 0.05; compared with preoperative parameters.
Prospective Study
Capogrosso et.al. (2023) on a single-institution prospective study (NCT03583034) performed at a tertiary referral centre that included 243 consecutive patients with lower urinary tract symptoms (LUTS) due to benign prostatic enlargement (BPE) treated with HoLEP by a single experienced surgeon (>1600 cases). Patients were assessed using validated questionnaires and uroflowmetry at baseline and several follow-up dates. Intraoperative and postoperative complications were recorded. Kaplan-Meier analysis was used to estimate recovery rates for urinary continence and erectile function. Logistic regression models were constructed to assess predictors of postoperative complications. Of the 243 patients, 78 (32.1%) had an indwelling urethral catheter. The median prostate volume (PV) was 87 cm3 (interquartile range 60-115) and 146 patients (59.8%) had PV >80 cm3. At 3-mo follow-up, 219 patients (90.1%) had a peak flow rate >20 ml/s and 182 (74.9%) had no postvoid residual urine. The improvement in subjective symptoms was significant at 1-mo follow-up and was maintained until 12 mo after surgery. Urinary continence recovery was slow, with an estimated rate of 68% (95% confidence interval [CI] 62-74%) at 1 mo and 94% (95% CI 91-97%) at 12 mo after HoLEP. The recovery rate for erectile function was 53% (95% CI 46-61%) at 1 mo and 85% (95% CI 77-90%) at 12 mo. Postoperative complications occurred in 36 patients (14.8%) during their hospital stay, in 34 (14%) within 1 mo following discharge from hospital, and in ten (4.1%) at later follow-up dates. Clinically significant complications (Clavien-Dindo ≥2) were observed in 44 cases (18%) and were more common for patients with an indwelling catheter at baseline (odds ratio 5.05; p = 0.006). It was conluded that HoLEP is an effective procedure for treating LUTS due to BPE, although it is not devoid of complications and sequelae, even in the hands of a highly experienced surgeon.
Summary of Evidence
For individuals who have benign prostatic hyperplasia and lower urinary tract symptoms who receive laser photo-vaporization of the prostate (PVP), the evidence includes a systematic review of randomized controlled trials and a retrospective study. The outcomes of interest are symptoms, quality of life, and treatment-related morbidity. The main treatment outcomes were measured by IPSS and IPSS Quality of Life Index (IPSS-QoL) at preoperative baseline, then at 1, 3, and up to 12 postoperative months (POM). Green-light laser photoselective vaporization of the prostate (PVP) had favorable outcomes in all the studies. Analysis revealed PVP is associated with reduced blood loss, transfusion, clot retention, Tur syndrome, capsular perforation, catheterization time, and hospitalization, but also with a higher reintervention rate and longer intervention duration. PVP was shown to have equivalent long-term IPSS, Qmax, QoL, PVR, IIEF efficacy, and fewer complications than TURP. PVP does not acquire histological tissue examination which removes an opportunity to identify prostate cancer and has a significantly higher incidence of reoperation for persistent/regrowth adenoma present. In the long-term period, showed a higher incidence of reoperation rate due to incomplete vaporization/regrowth of prostatic adenoma. Compared to HoLEP, Qmax and PVR improved significantly compared to baseline beginning 1 month after surgery in both the PVP and HoLEP groups; this improvement was sustained throughout the 12 months of follow-up. In another study, the increase in the Qmax value 60 months after the PVP was decreased to the baseline level, but there's still a risk of re-operation. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome.
| Population Reference No. 2 Policy Statement | [X] Medically Necessary | [ ] Investigational |
The purpose of the following information is to provide reference material. Inclusion does not imply endorsement or alignment with the evidence review conclusions.
While the various physician specialty societies and academic medical centers may collaborate with and make recommendations during this process, through the provision of appropriate reviewers, input received does not represent an endorsement or position statement by the physician specialty societies or academic medical centers, unless otherwise noted.
American Urological Association (AUA) statements for 2021:
"Surgery is recommended for patients who have renal insufficiency secondary to BPH, refractory urinary retention secondary to BPH, recurrent urinary tract infections (UTIs), recurrent bladder stones or gross hematuria due to BPH, and/or with LUTS/BPH refractory to or unwilling to use other therapies. (Clinical Principle)"
“Holmium laser enucleation of the prostate (HoLEP) or thulium laser enucleation of the prostate (ThuLEP) should be considered as an option, depending on the clinician’s expertise with these techniques, as prostate size-independent options for the treatment of LUTS/BPH. (Moderate Recommendation; Evidence Level: Grade B)”
“HoLEP, PVP, and ThuLEP should be considered as treatment options in patients who are at higher risk of bleeding. (Expert Opinion)”
"PVP should be offered as an option using 120W or 180W platforms for the treatment of LUTS/BPH. (Moderate Recommendation; Evidence Level: Grade B)"
European Association of Urology (EAU) statements for 2023:
5.3.2.3. Holmium laser enucleation of the prostate
5.3.3.2.532 nm (‘Greenlight’) laser vaporization of the prostate
National Institute for Health and Care Excellence (NICE)
"If offering surgery for managing voiding LUTS presumed secondary to BPE, offer monopolar or bipolar transurethral resection of the prostate (TURP), monopolar transurethral vaporization of the prostate (TUVP) or holmium laser enucleation of the prostate (HoLEP). Perform HoLEP at a center specializing in the technique, or with mentorship arrangements in place. [2010]"
HoLEP Guidance (2003):
“Current evidence on the safety and efficacy of holmium laser prostatectomy appears adequate to support the use of the procedure, provided that normal arrangements are in place for consent, audit, and clinical governance.”
“Clinicians undertaking this procedure require specialist training. The British Association of Urological Surgeons has agreed to produce training standards.”
GreenLight Guidance (2022):
"GreenLight XPS is recommended as an option to treat benign prostatic hyperplasia (BPH) in adults."
" Data should continue to be collected on cost-saving outcomes for GreenLight XPS compared with other treatments in people who may be considered high risk. This includes people with larger prostates and a higher risk of bleeding."
There is no national coverage determination. In the absence of a national coverage determination, coverage decisions are left to the discretion of local Medicare carriers.
| Codes | Number | Description |
| CPT | 52648 | Laser vaporization of prostate, including control of postoperative bleeding, complete (vasectomy, meatotomy, cystourethroscopy, urethral calibration and/or dilation, internal urethrotomy and transurethral resection of prostate are included if performed) |
| 52649 | Laser enucleation of the prostate with morcellation, including control of postoperative bleeding, complete (vasectomy, meatotomy, cystourethroscopy, urethral calibration and/or dilation, internal urethrotomy and transurethral resection of prostate are included if performed) | |
| ICD - 10 - CM | D29.1 | Benign neoplasm of prostate |
| N40.1 | Benign prostatic hyperplasia with lower urinary tract symptoms | |
| N40.3 | Nodular prostate with lower urinary tract symptoms | |
| Type of service | Surgery | |
| Place of service | Inpatient/ Outpatient |
| Date | Action | Description |
| 10/24/2024 | Annual Review | Policy updated with literature review through September 30, 2024. References added. Policy statements unchanged. Reviewed and Approved by Physician Advisory Commitee. |
| 10/26/2023 | Annual Committee Review | Reviewed by the Providers Advisory Committee. No changes on policy statement. |
| 07/14/2023 | Policy Creation | The use of laser prostatectomy procedures (HoLEP, PVP) in individuals with moderate-to-severe lower urinary tract obstruction due to benign prostatic hyperplasia as an alternative to open prostatectomy or transurethral resection of the prostate is considered medically necessary. |